Translocation t(11;14) (q13;q32) is a specific genetic marker of the mantle cell lymphomas (MCL). Recent studies suggest that this translocation is present in almost all MCL. Except for MCL, t(11;14) translocation has been exceptionally described also in different lymphomas and leukemia from which it is being relatively more frequently demonstrated in the multiple myeloma.
When chromosomal translocation t(11;14) (q13;q32) it comes to a fusion of the joining region of the gene for the heavy chain of immunoglobuline (IgH) on the chromosome 14 and noncoding region of the gene bcl-1 (cyklin D1 (CCND1), prad1) on the chromosome 11. In this case of fusion the gene for cyclin D1 gets under the influence of the IgH gene. As a consequence of this it comes to the overexpression of the cyclin D1 and so to the disruption of the central cell cycle pathway balance. Breakages in the locus blc-1 occure in 30 - 55 % of cases in the major translocation cluster (MTC) but in 10 - 20 % of cases also other regions distally from MTC were identified.
The translocation t(11;14) (q13;q32) and overexpression of the cycline D1 are relatively easily detectable by the molecular biological and cytogenetic methods. The results of these examinations allow for more accurate and reliable diagnosis especially in cases when only on the basis of morphology and immunohistochemistry it is difficult to distinguish MCL from chronic lymphocytic leukemia, follicular lymphoma or marginal zone lymphoma. Therefore, they have a crucial influence on the next treatment and thus on the fate of the patient.
Examination
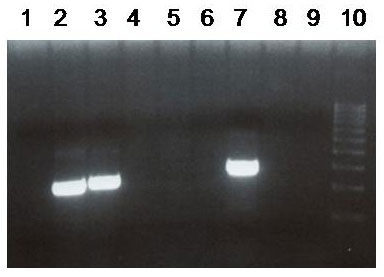
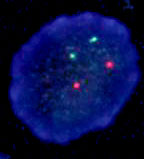
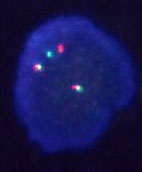
There have been a number of procedures developed to detect translocation t(11;14)(q13;q32). Currently, the most used methods are apparently that which are based on PCR (polymerase chain reaction) and FISH (fluorescence in-situ hybridisation). In our laboratory we use two-round PCR with a set of primers overlapping the MTC region. In the case of the positive result these primers are amplified depending on the location of the break. The amplified fragments are of the length about 200 - 300 bp and are detectable by the agarose gel electrophoresis (Fig. 1). For the interphase FISH we use the commercially available probe IgH/CCND1 Dual Color, Dual Fusion Translocation Probe from the Vysis/Abbott company. With this probe we get two red and two green signals in the normal nucleus (fig. 2a). In the sample with positive translocation t(11;14) (q13;q32) there are two yellow fused signals from translocated chromosomes and one red and one green signal from intact chromosomes (fig. 2b).
References
- Lasota J, Franssila K, Koo CH, Miettinen M. Molecular diagnosis of mantle cell lymphoma in paraffin-embedded tissue. Mod Pathol. 1996;9(4):361-6.
- de Boer CJ, van Krieken JH, Schuuring E, Kluin PM. Bcl-1/cyclin D1 in malignant lymphoma. Ann Oncol. 1997;8 Suppl 2:109-17.
- Li JY, Gaillard F, Moreau A, Harousseau JL, Laboisse C, Milpied N, Bataille R, Avet-Loiseau H. Detection of translocation t(11;14)(q13;q32) in mantle cell lymphoma by fluorescence in situ hybridization. Am J Pathol. 1999;154(5):1449-52.
- World Health Organisation Classification of Tumours: Pathology and Genetics. Tumours of Haematopoietic and Lymphoid Tissues. Ed. Jaffe, ES, Harris, NL, Stein, H, Vardiman JW. IARC Press, Lyon, 2001.